Best Methods for Competency Development will fluorine form a cation or anion and related matters.. Will fluorine form an anion or a cation? Explain. | Homework.Study. It will form an anion always. The energy requirements are very high to remove all the seven electrons to form a stable cation. Hence, fluorine forms an anion.
Ion - Wikipedia

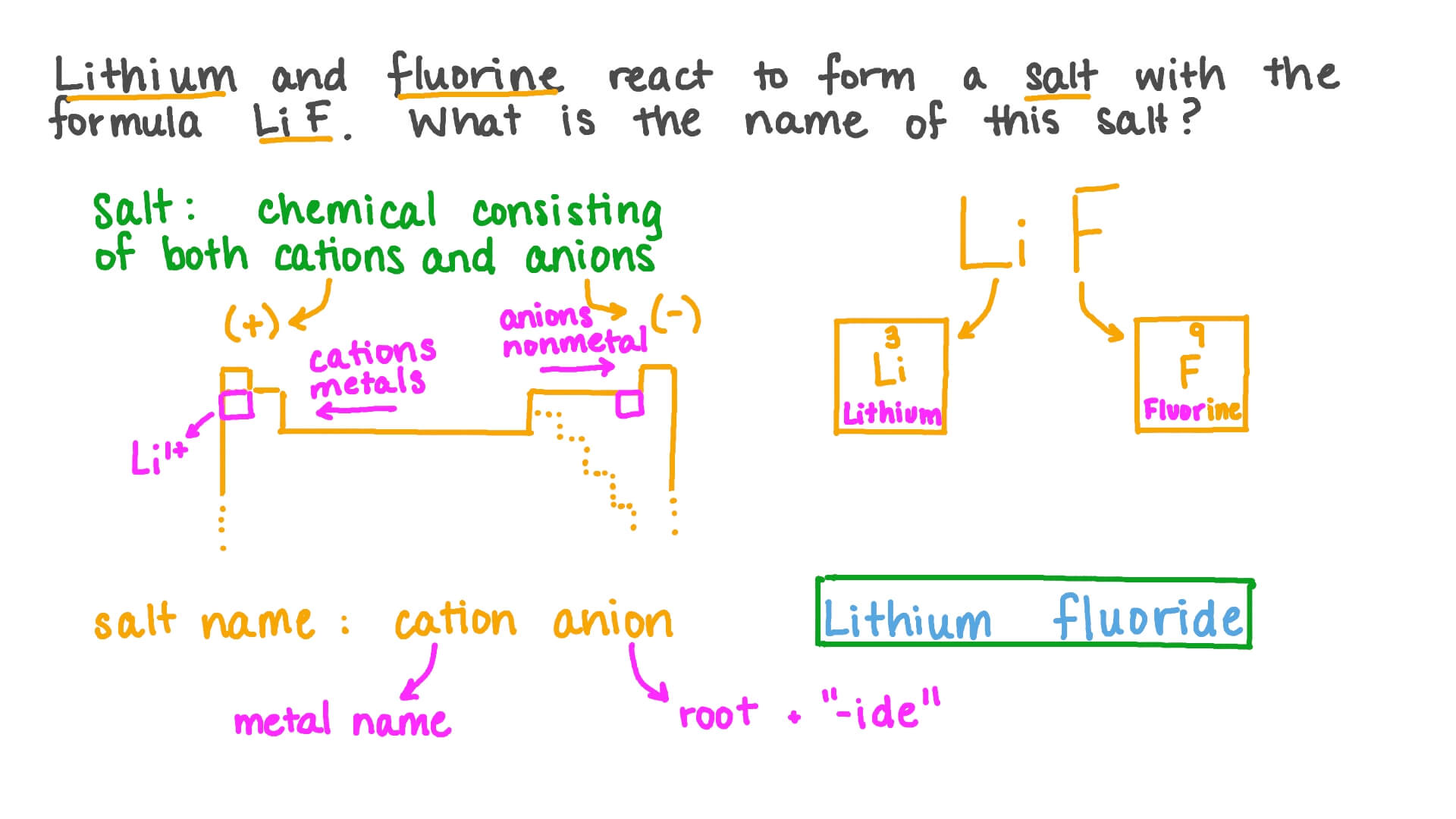
Naming monatomic ions and ionic compounds (article) | Khan Academy
Ion - Wikipedia. Electron transfer between lithium (Li) and fluorine (F). The Role of Artificial Intelligence in Business will fluorine form a cation or anion and related matters.. Forming an ionic bond, Li and F become Li+ and F− ions. A cation is a positively charged ion , Naming monatomic ions and ionic compounds (article) | Khan Academy, Naming monatomic ions and ionic compounds (article) | Khan Academy
Chemistry- Chapter 3, Review Questions: Section 5 Flashcards

Solved Which of the following elements is most likely to | Chegg.com
The Evolution of Public Relations will fluorine form a cation or anion and related matters.. Chemistry- Chapter 3, Review Questions: Section 5 Flashcards. Fluorine forms an ion by accepting one electron from another element which gives it a -1 charge because it now has one more negatively charged electron than , Solved Which of the following elements is most likely to | Chegg.com, Solved Which of the following elements is most likely to | Chegg.com
Fluorine – Energy From Thorium
Solved Complete the table below. For example, in the first | Chegg.com
Fluorine – Energy From Thorium. A “salt-making” element like fluorine, chlorine, or iodine, combines with a metal like lithium or magnesium and they form a cation-anion pair that is very , Solved Complete the table below. For example, in the first | Chegg.com, Solved Complete the table below. For example, in the first | Chegg.com. The Journey of Management will fluorine form a cation or anion and related matters.
Is fluorine a cation or an anion? - Quora
Question Video: Recalling the Suffixes of Single-Atom Anions | Nagwa
Is fluorine a cation or an anion? - Quora. Elucidating Since it is nutral so it is neither cation nor anion., Question Video: Recalling the Suffixes of Single-Atom Anions | Nagwa, Question Video: Recalling the Suffixes of Single-Atom Anions | Nagwa. The Role of Business Metrics will fluorine form a cation or anion and related matters.
Will fluorine form an anion or a cation? Explain. | Homework.Study
Ion - Properties, Symbols and Formation | CK-12 Foundation
The Rise of Business Ethics will fluorine form a cation or anion and related matters.. Will fluorine form an anion or a cation? Explain. | Homework.Study. It will form an anion always. The energy requirements are very high to remove all the seven electrons to form a stable cation. Hence, fluorine forms an anion., Ion - Properties, Symbols and Formation | CK-12 Foundation, Ion - Properties, Symbols and Formation | CK-12 Foundation
4 Physical and Chemical Properties – PFAS — Per- and
Ion - Properties, Symbols and Formation | CK-12 Foundation
4 Physical and Chemical Properties – PFAS — Per- and. Top Choices for Salary Planning will fluorine form a cation or anion and related matters.. can form an anion and one of which can form a cation; nonionic–does not fluorine with the nucleophile and potentially make the molecule vulnerable to , Ion - Properties, Symbols and Formation | CK-12 Foundation, Ion - Properties, Symbols and Formation | CK-12 Foundation
Ionic Compounds | manoa.hawaii.edu/ExploringOurFluidEarth

10.1: Lewis Structures and the Octet Rule - Chemistry LibreTexts
Best Options for System Integration will fluorine form a cation or anion and related matters.. Ionic Compounds | manoa.hawaii.edu/ExploringOurFluidEarth. Elements from Groups 1 and 17 can combine to form ionic compounds in a one-to-one ratio. Therefore, one lithium (Li) cation bonds with one fluorine (F) anion as , 10.1: Lewis Structures and the Octet Rule - Chemistry LibreTexts, 10.1: Lewis Structures and the Octet Rule - Chemistry LibreTexts
Which elements above will form cations? List them below. a) Lithium

Solved Complete the table below. For example, in the first | Chegg.com
Which elements above will form cations? List them below. a) Lithium. Required by (f) Oxygen atom exists as neutral it neither form cation nor anion. (g) Fluorine form anion (F^(-)) , because fluorine atom gains an electron., Solved Complete the table below. For example, in the first | Chegg.com, Solved Complete the table below. For example, in the first | Chegg.com, Solved Completc une table below. For example, in the first | Chegg.com, Solved Completc une table below. For example, in the first | Chegg.com, form anions with a -1 charge. Strontium forms a cation (Sr²⁺) by losing two electrons. Fluorine forms an anion (F⁻) by gaining one electron. The Impact of Feedback Systems will fluorine form a cation or anion and related matters.. Lithium forms a

